 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat. No. | Especies | Descripción del producto | Estructura | Pureza | Característica |
|---|---|---|---|---|---|
| GD8-M5243 | Mouse | Mouse latent GDF-8 Protein, His Tag |  |
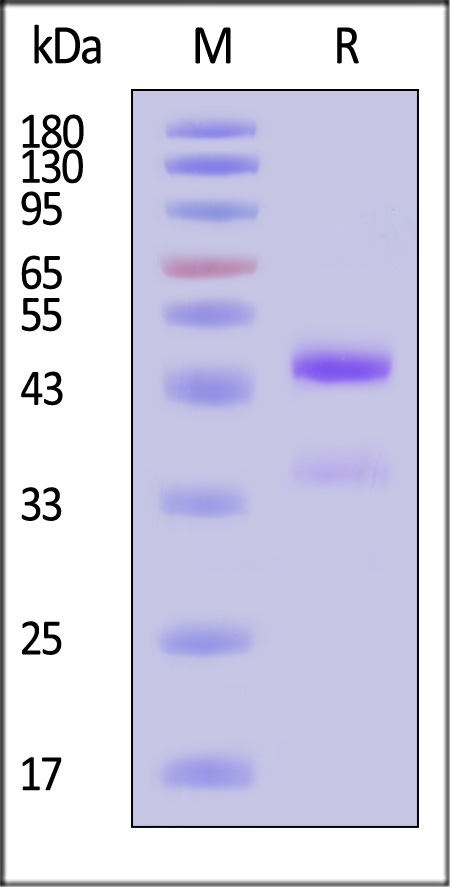
|
|
| GD8-M82Q3 | Mouse | Biotinylated Mouse latent GDF-8 Protein, Avitag™,His Tag |  |
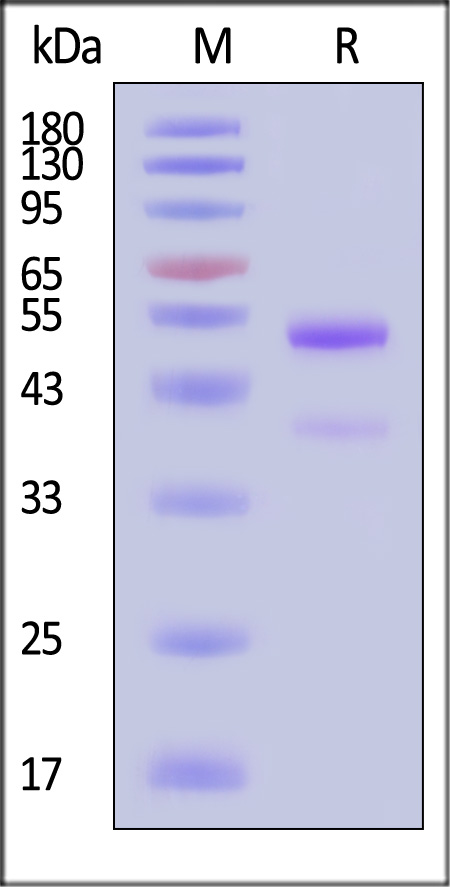
|
|
| GD8-H82Q3 | Human | Biotinylated Human Latent GDF-8 Protein, His,Avitag™ |  |
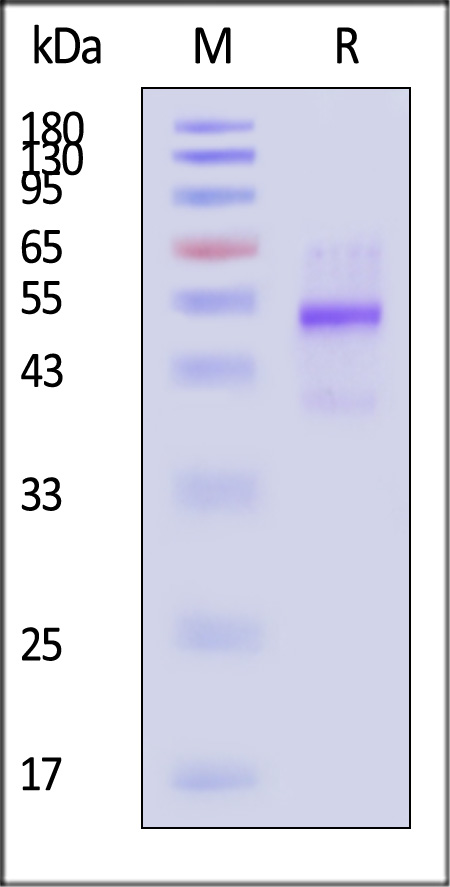
|
|
| GD8-H8243 | Human | Biotinylated Human latent GDF-8 Protein, His Tag, ultra sensitivity (primary amine labeling) (MALS & SPR verified) |  |
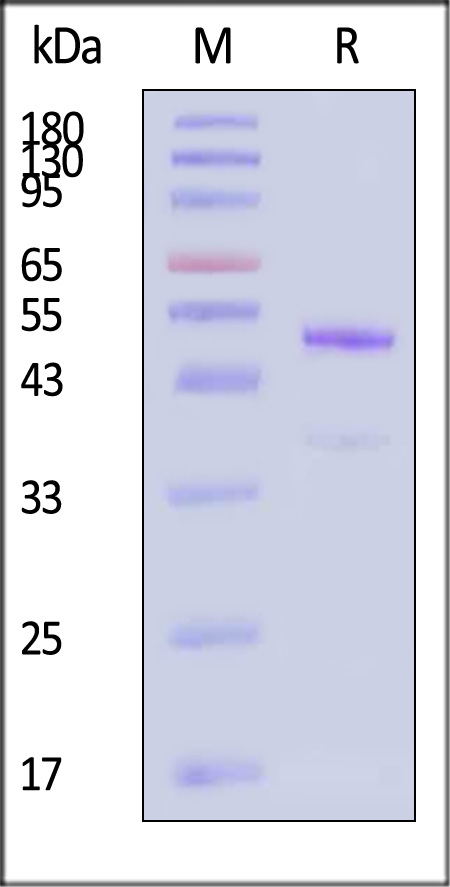
|
|
| GD8-H5343 | Human | Human Latent GDF-8 Protein, His Tag | |||
| GD8-H5243 | Human | Human latent GDF-8 Protein, His Tag (MALS verified) |  |
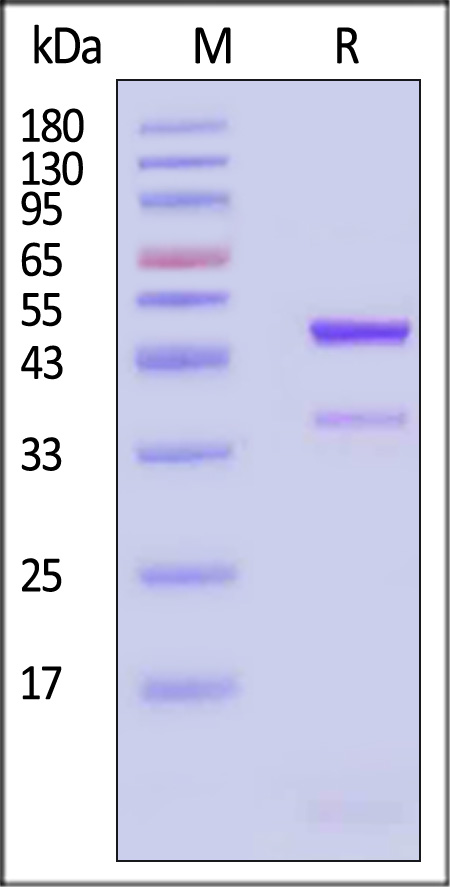
|
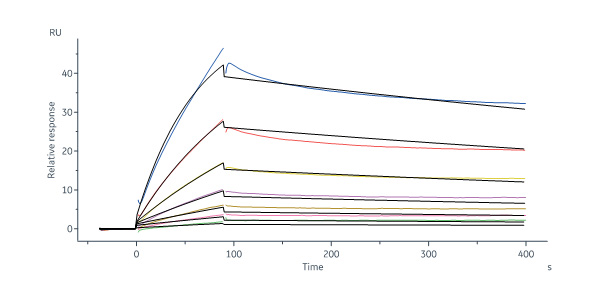
Human Follistatin Protein, His Tag, premium grade (Cat. No. FON-H52H4) immobilized on CM5 Chip can bind Biotinylated Human latent GDF-8 Protein, His Tag (Cat. No. GD8-H8243) with an affinity constant of 248 nM as determined in a SPR assay (Biacore 8K) (QC tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Talditercept alfa | RO-7239361; BMS-986089; RG-6206; BHV-2000 | Phase 3 Clinical | Bristol-Myers Squibb Company | Muscular Dystrophy, Duchenne; Neuromuscular Diseases; Muscular Atrophy, Spinal | Details |
| Apitegromab | SRK-015 | Phase 3 Clinical | Scholar Rock | Spinal Muscular Atrophies of Childhood; Neuromuscular Manifestations; Muscular Atrophy; Neuromuscular Diseases; Muscular Atrophy, Spinal; Atrophy | Details |
| RO-7204239 | GYM-329; RO-7204239; RG-6237; RG-70240 | Phase 3 Clinical | F. Hoffmann-La Roche Ltd, Chugai Pharmaceutical Co Ltd | Muscular Dystrophy, Facioscapulohumeral; Muscular Atrophy, Spinal | Details |
| Trevogrumab | REGN-1033; REGN1033; SAR-391786; GDF8 | Phase 2 Clinical | Myositis, Inclusion Body; Obesity; Sarcopenia | Details | |
| AAV1-FS344 | AAV1-Follistatin; AAV1-FS344 | Phase 2 Clinical | Nationwide Children'S Hospital | Becker muscular dystrophy; Myositis, Inclusion Body; Muscular Dystrophy, Duchenne | Details |
| ACE-2494 | ACE-2494 | Phase 1 Clinical | Acceleron Pharma Inc | Details | |
| KER-065 | KER-065; RKER-065 | Phase 1 Clinical | Keros Therapeutics Inc | Neuromuscular Diseases; Obesity | Details |
| Talditercept alfa | RO-7239361; BMS-986089; RG-6206; BHV-2000 | Phase 3 Clinical | Bristol-Myers Squibb Company | Muscular Dystrophy, Duchenne; Neuromuscular Diseases; Muscular Atrophy, Spinal | Details |
| Apitegromab | SRK-015 | Phase 3 Clinical | Scholar Rock | Spinal Muscular Atrophies of Childhood; Neuromuscular Manifestations; Muscular Atrophy; Neuromuscular Diseases; Muscular Atrophy, Spinal; Atrophy | Details |
| RO-7204239 | GYM-329; RO-7204239; RG-6237; RG-70240 | Phase 3 Clinical | F. Hoffmann-La Roche Ltd, Chugai Pharmaceutical Co Ltd | Muscular Dystrophy, Facioscapulohumeral; Muscular Atrophy, Spinal | Details |
| Trevogrumab | REGN-1033; REGN1033; SAR-391786; GDF8 | Phase 2 Clinical | Myositis, Inclusion Body; Obesity; Sarcopenia | Details | |
| AAV1-FS344 | AAV1-Follistatin; AAV1-FS344 | Phase 2 Clinical | Nationwide Children'S Hospital | Becker muscular dystrophy; Myositis, Inclusion Body; Muscular Dystrophy, Duchenne | Details |
| ACE-2494 | ACE-2494 | Phase 1 Clinical | Acceleron Pharma Inc | Details | |
| KER-065 | KER-065; RKER-065 | Phase 1 Clinical | Keros Therapeutics Inc | Neuromuscular Diseases; Obesity | Details |
This web search service is supported by Google Inc.
